
Addressing Out Of
Control Prescription
Drug Prices
Federal and State Strategies
May 2018
Report authored by
Quynh Chi Nguyen, Policy Analyst
Michael Miller, Strategic Policy Director

Addressing Out Of Control
Prescription Drug Prices
Federal and State Strategies
Addressing Out Of Control
Prescription Drug Prices
Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
2
Community Catalyst is committed to improving prescription drug policies to
ensure that consumers have access to safe, affordable and appropriate drugs.
Prescription drug pricing, utilization and quality are part of the overall reforms
that must be made to the delivery system to increase the value of health care.
Since 2001, Community Catalyst has been a leading independent consumer
voice on a wide range of prescription drug issues. Unlike many other consumer
organizations, we do not get support from the industry. Our Prescription Access
Litigation (PAL) project supported class action lawsuits by the private bar that
challenged illegal industry practices, resulting in $1 billion in awards to
consumers and health plans, including union health and welfare funds.
In 2007, Community Catalyst, with the support of the Pew Charitable Trusts,
launched the Prescription Project, which led state and federal public policy
campaigns to pass pharmaceutical marketing transparency laws in several
states, culminating in the Physician Payment Sunshine Act included as part
of the Affordable Care Act. The project’s efforts to expose conflicts of interest
between prescribers and the pharmaceutical and medical device industries
also compelled dozens of academic medical centers across the country to
revise and strengthen their conflict-of-interest policies. The work of the
Prescription Project also drove passage of state legislation and federal
programs to increase unbiased physician education on drug effectiveness.
Community Catalyst has built a state consumer health advocacy infrastructure
with a strong track record of success in achieving policy and system changes
that improve access and quality of care for all consumers, especially vulnerable
populations. State advocates effectively operate at the local and federal levels,
bringing their grounded experience and informed consumer voice to key health
care decision-making arenas. Changes in the organization and financing of
medical care since the Affordable Care Act have created new opportunities to
address current deficiencies in the delivery of medical care. In this context,
Community Catalyst and its Center for Consumer Engagement in Health
Innovation are working to build the voice of consumers and communities, to
achieve better health for all.
Acknowledgement
We would like to extend thanks to our external reviewers—Jerry Avorn, Professor
of Medicine at Harvard Medical School and Chief of the Division
of Pharmacoepidemiology and Pharmacoeconomics in the Department of
Medicine at Brigham and Women’s Hospital; Celia Segel, Director of
Comparative Effectiveness Research Policy Development at the Institute
for Clinical and Economic Review; and Anthony So, Director of the Johns
Hopkins Center for a Livable Future—who took time out of their very busy
schedules to provide perceptive comments that made significant contributions
to development of this paper. The views expressed here do not necessarily
reflect the views of our external reviewers.

Table of Contents
Table of Contents
Executive Summary ..........................................................................................................4
Introduction ...................................................................................................................11
Impact of Rapidly Increasing Prescription
Drug Costs on Consumers and the Government .................................................................12
Key Factors Contributing to Rapidly Increasing Prescription
Drug Costs and the Lack of Affordability for Consumers ....................................................13
•
Problem 1: Pharmaceutical monopoly power over drug pricing ........................13
Patent and market exclusivity rights ..........................................................13
Anticompetitive practices ........................................................................13
Lack of federal and state authority to negotiate lower drug prices ................14
•
Problem 2: The opaque pharmaceutical supply chain .....................................14
Lack of transparency and granularity of
information on drug pricing decisions .......................................................14
Pharmacy benefit managers’ game-playing ................................................15
•
Problem 3: Manipulative marketing tactics ...................................................15
•
Problem 4: Insurers shifting costs to consumers ............................................16
High out-of-pocket cost sharing ................................................................ 16
Discriminatory formulary designs (adverse tiering) ......................................16
A Policy Framework for Federal and State Actions ...........................................................17
•
Solution 1: Enact legislation and leverage existing
federal authorities to reduce pharmaceutical monopoly
power over drug pricing ...............................................................................17
•
Solution 2: Enact legislation that mandates public
disclosure on drug pricing, investment in drug development,
manufacturing and marketing in order to break down the
opaque pharmaceutical supply chain ............................................................ 20
•
Solution 3: Enact legislation that prohibits manipulative
marketing practices that lure providers and consumers
toward more expensive alternatives...............................................................21
•
Solution 4: Enact legislation and regulations that aim to
reduce cost sharing and prohibit discriminatory formulary
designs (known as adverse tiering) to ensure equitable access
to affordable medications ............................................................................21
Conclusion ....................................................................................................................22
Glossary ........................................................................................................................23
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
3

Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
Executive Summary
The practice of granting patent monopolies to pharmaceutical companies to spur
innovation is arguably a flawed approach to advancing continuous innovation of safe,
effective and affordable medicines. Even in the context of this flawed paradigm, the
United States (U.S.) does poorly relative to other countries at making prescription drugs
affordable for its population. Prescription drug prices and spending are consistently much
higher in the U.S. than in other high-income countries. Studies show Americans pay at
least three times more for prescription drugs than residents in other high-income
countries.
High prescription drug prices are a growing concern for many Americans: One in four of
those taking a prescription drug reported skipping doses or cutting pills in half due to
costs. For millions of Americans with chronic conditions, access to needed medications
has been a persistent issue.
The majority of Americans across the political spectrum are demanding that Congress
and the Trump administration take action to lower prescription drug costs. Federal
policymakers are better positioned than those at the state level to drive down drug prices.
However, it is unclear whether Congress and the current administration are willing to
tackle the problem in an effective way at any time soon. Therefore, to make progress on
prescription drug affordability, states need to lead the way.
This brief highlights four key factors contributing to the high cost of prescription drugs in
the U.S. including:
• Problem 1: Pharmaceutical monopoly power over drug pricing that is not
counterbalanced by a strong coordinated purchasing strategy as it is in most
high-income countries.
• Problem 2: The opaque pharmaceutical supply chain that allows various
intermediary players to maximize their profits.
• Problem 3: Manipulative marketing tactics that drug manufacturers use to lure
providers and consumers toward high-cost medications.
• Problem 4: Insurers shifting costs to consumers that leads to unaffordable
medications millions of people depend on.
In addition, we include a policy framework that can guide actions at the federal and state
levels to tackle each of the identified problems. Depending upon the political environment
and resources in each state, consumer advocates, policymakers and stakeholders can
work together to adopt a variety of measures that help curb unfair drug pricing. We hope
our recommendations are valuable to consumer advocates and other stakeholders with
a shared interest in taking practical steps to ensure equitable access to affordable and
effective medicines for all. While this work requires challenging a strong pharmaceutical
lobby, effective advocacy strategies and strong grassroots support can put victories
within reach.
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
4

• Targeting physicians
with gifts and other
incentives
• Heavily investing in
prescription drug
advertising & marketing
to steer patients away
from lower-cost
medications
• Strategic granting to
influence
disease-specific patient
groups
• Patent and market
exclusivity rights
• Anticompetitive
practices
• Lack of federal and
state authority to
negotiate lower drug
prices
• Opportunities for
profit-taking among
various intermediary
players (including
wholesalers, pharmacy
benefit managers,
retailers and insurers)
The Results?
Rising costs of prescription drugs disproportionately harm
low-income people and people living with chronic conditions
Pharmaceutical
Monopoly Power
Lack of Transparancy
in the Supply Chain
Manipulative
Pharmaceutical
Marketing
Insurers Shifting
Costs to Consumers
• Placing most or all
drugs that treat a specific
condition on the highest
cost-sharing tiers to
discourage sick people
from enrolling
• High coinsurance and
copays
One out of five Americans taking prescription drugs either
skips doses or cuts pills due to costs
Explaining the Sky-High
Cost of Prescription Drugs
5
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
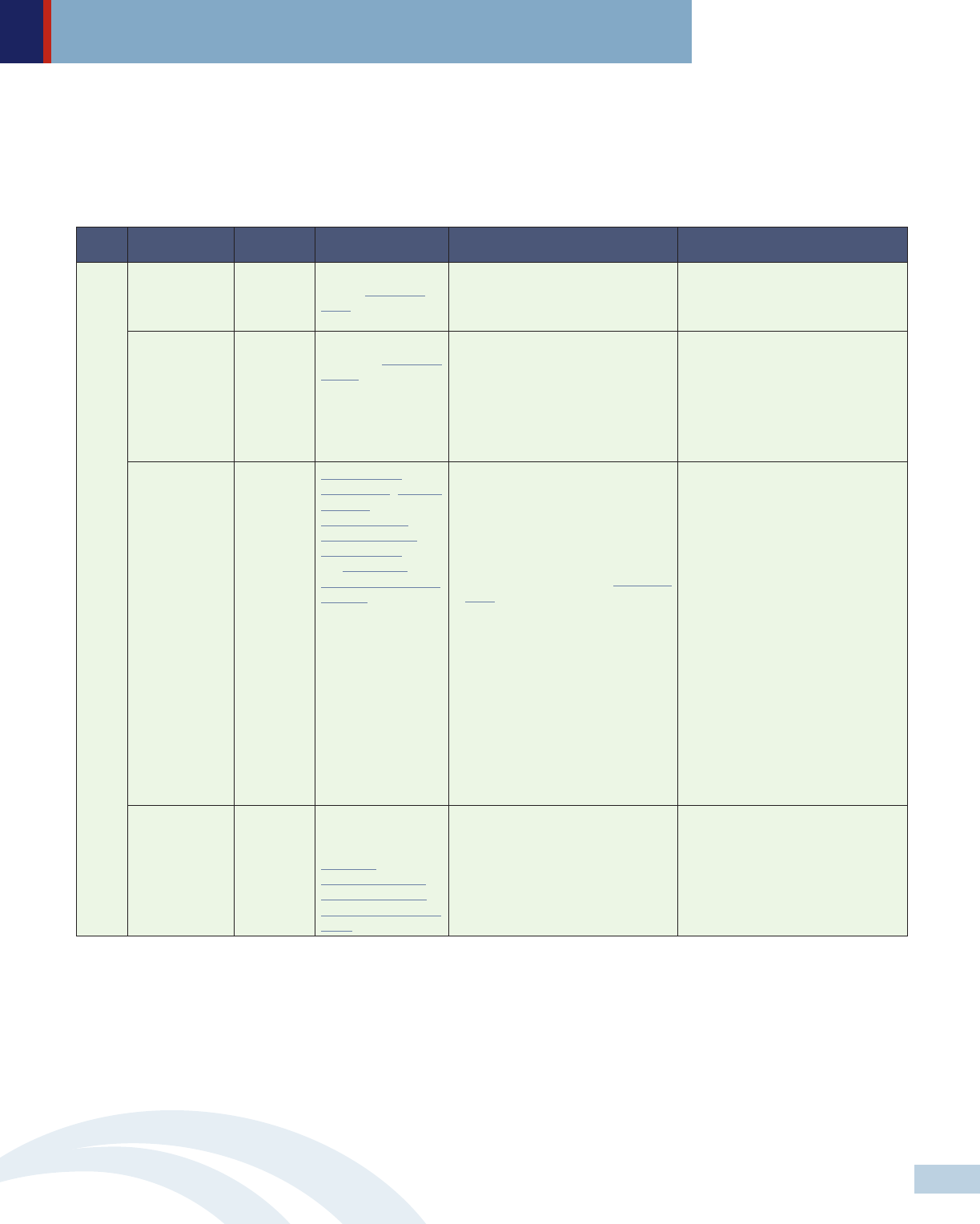
Level Policymakers Approach Policy strategies Specific recommendations Selected examples
HHS Regulation Leverage March-In
Rights (35 U.S.C.
§203)
• Force patent manufacturers that
receive federal grants for research
and development to allow generic
drugs to enter the market.
HHS Regulation Leverage Patent &
Copyright (28 U.S.C.
§1498)
• License generic versions of high-cost
medications at low prices.
Early 2017, LA’s Department of
Health considered using 28 U.S.C. §
1498 to by-pass Gilead’s patents on
Sovaldi and Harvoni, two of the new,
highly effective hepatitis C drugs, to
treat people with hepatitis C in the
state’s Medicaid program, prison
system, and its uninsured
Congress Legislation Amend Hatch-
Waxman Act, Orphan
Drug Act, the
Biologics Price
Competition and
Innovation Act, and
the Generating
Antibiotic Incentives
Now Act.
• Shorten patent and market
exclusivity periods and eliminate
patent extensions for drugs that
have no demonstrated added value
compared to those already on the
market;
• Amend ‘March-In Rights’ (35 U.S.C.
§203) to set limits on introductory
prices for new innovative drugs and
annual price increases for existing
drugs that have received federal
funding for research and
development; and
• Increase FTC resources to monitor,
oversee and investigate drug
manufacturers engaging in
anticompetitive practices and
empower the FDA to terminate
market exclusivity on any product
found to be in violation.
Congress Legislation Eliminate the
‘noninterference’
clause in the
Medicare
Prescription Drug,
Improvement, and
Modernization Act of
2003
• Allow Medicare to directly negotiate
drug price with drug manufacturers;
and
• Allow HHS to limit what Medicare
pays for drug based on price of
another therapeutically equivalent
drug.
A policy framework for federal and state actions
• Solution 1: Enact legislation and leverage existing federal authorities that aim to reduce pharmaceutical monopoly power
over drug pricing. Policymakers should put in place policies that enable vigorous and effective competition that will
bring down drug prices.
Federal
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
6
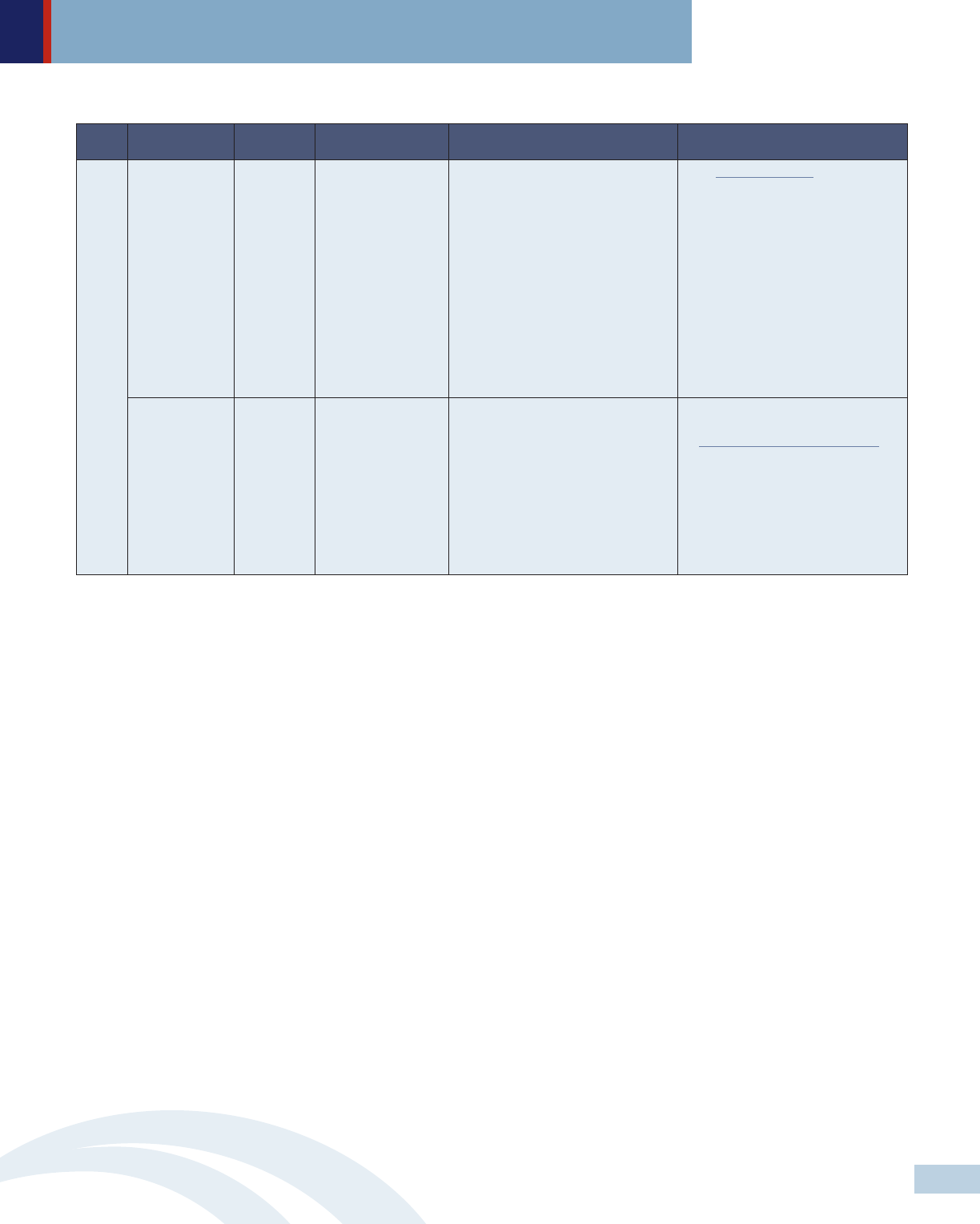
Level Policymakers Approach Policy strategies Specific recommendations Selected examples
Legislature Legislation Enact legislation that
prohibits price
gouging for all drugs
• Require drug manufacturers to
justify price increases or face
substantial penalties.
MD’s price gouging law authorizes the
state AG to prosecute drug
manufacturers that engage in
excessive price increases in
noncompetitive off-patent or generic
drug markets. A three judge panel of
the U.S. Court of Appeals for the
Fourth Circuit recently ruled 2-1 that
MD’s anti-price gouging law
unconstitutionally violates the
“Dormant Commerce Clause,”
because, in their view, it would affect
transactions in other states. Maryland
AG has appealed the ruling to the full
Fourth Circuit Court of Appeals.
Department of
health or
relevant
agencies
State plan
amendment
Establish multistate
and/or in-state
collaborative
• Establish multistate purchasing
pools of high cost medications to
negotiate reduced prices; and
• Operate as a PBM to represent
in-state participants that use unified
formularies for all covered members
across state and local programs.
More than half of the states are
currently participating in at least one
of four multistate purchasing pools. In
addition to the federally required
rebate, drug manufacturers offer these
states supplemental rebates.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
State
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
7
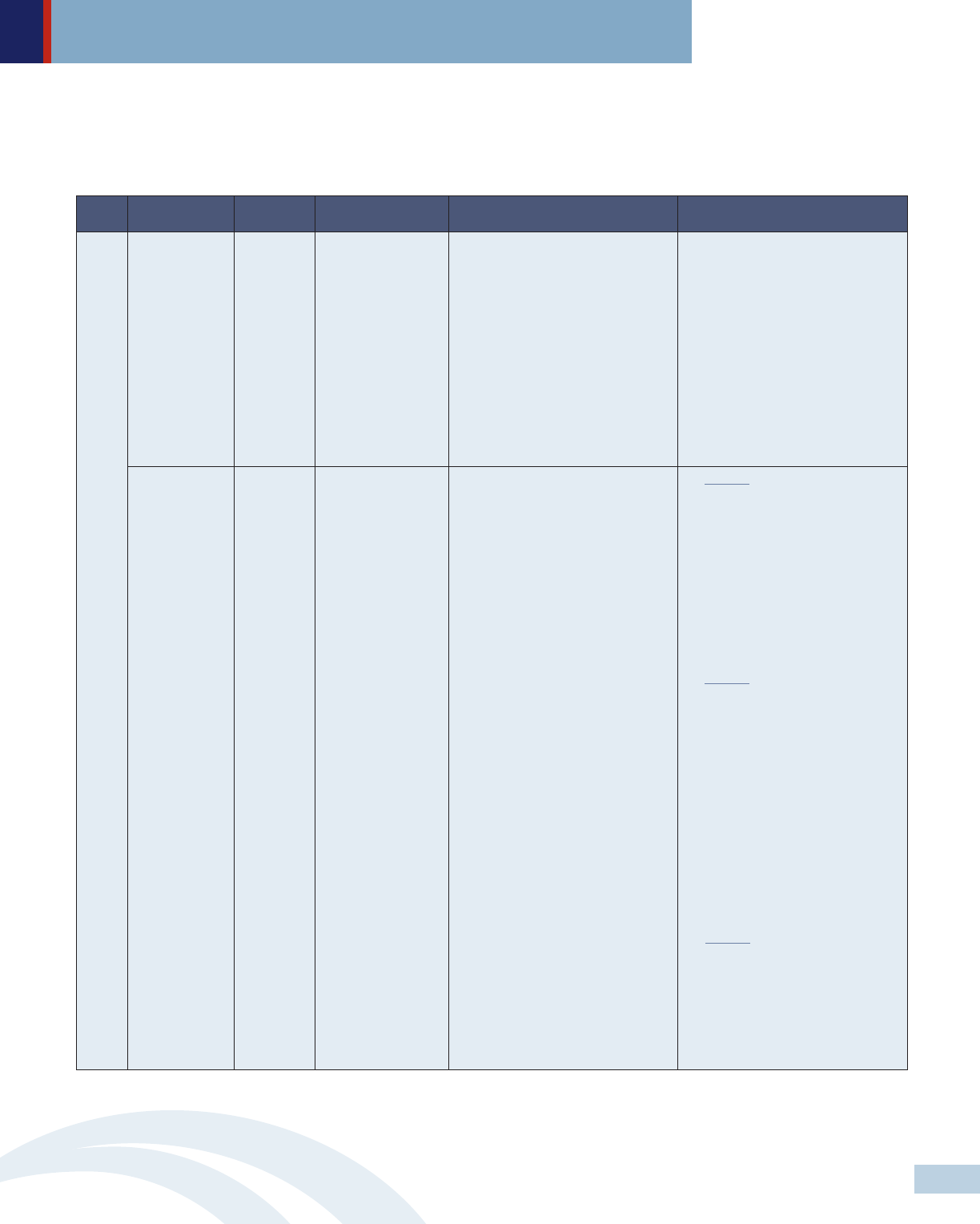
• Solution 2: Enact legislation that mandates public disclosure on drug pricing, investment in drug development, manufacturing
and marketing to create transparency within the pharmaceutical supply chain. Detailed information on drug pricing and
clinical efficacy should be available to the public in a clear, straightforward and timely manner.
Level Key players Approach Policy strategies Specific recommendations Selected examples
Legislature Legislation Create a drug price
review commission
A drug price review commission
should:
• Include at least 2 members
representing patients and health
care consumers as well as
representatives from health care
providers, public-employer-and-
commercial payers and researchers;
• Have the authority to approve or
reject drug price setting; and
• Offer public input opportunities
during review periods.
MD has filed a bill based on NASHP’s
Model Drug Price Transparency Act to
evaluate the affordability of certain
drugs and impose limits on how much
the state and commercial health plans
will pay.
Legislature Legislation Enact drug price
transparency
legislation
Require department of health or
relevant agencies to set a price cap for
a particular drug at a fixed percentage
above the average price for that drug
sold in OECD countries.
Require drug manufacturers to:
• Submit justifications for all drugs
that have a 10% price increase
above the previous price and to
undergo a price review process;
• Provide advance notices of price
hikes to give time for purchasers to
adjust formularies, negotiate price
concessions or seek other
alternatives; and
• Publicly disclose detailed
information on prescription drug
pricing as well as development,
manufacturing and marketing costs
on a drug-by-drug basis and grants
to non-profit groups.
Require PBMs and nonprofit groups to
submit reports of their financial
arrangements with pharmaceutical
manufacturers.
Require insurers to show how increase
in prescription drug prices affect
premiums.
NV requires diabetes drug
manufacturers to disclose information
about the costs of making and
marketing drugs where prices increase
by a certain amount. PBMs and
nonprofit groups receiving
pharmaceutical grants are also
required to submit annual reports to
the state department of health and
human services of their financial
arrangements with drug
manufacturers.
NY requires the state Department of
Health to establish a Medicaid
spending cap with year to year
spending targets and to review drug
expenditures quarterly. If expenditures
exceed the department’s spending
cap, the commissioner identifies and
refers specific drugs to a drug
utilization board for recommended
supplemental rebates.
In addition to public disclosure
requirements, CA requires drug
manufacturers to provide a 60-day
advance notice of price increases for
most prescription drugs.
OR requires drug manufacturers to
report research and development and
marketing costs, profits and other
factors that contribute to a specific
drug’s price increase of more than
10%. OR also requires insurers to
show how high drug prices affect
premiums.
State
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
8
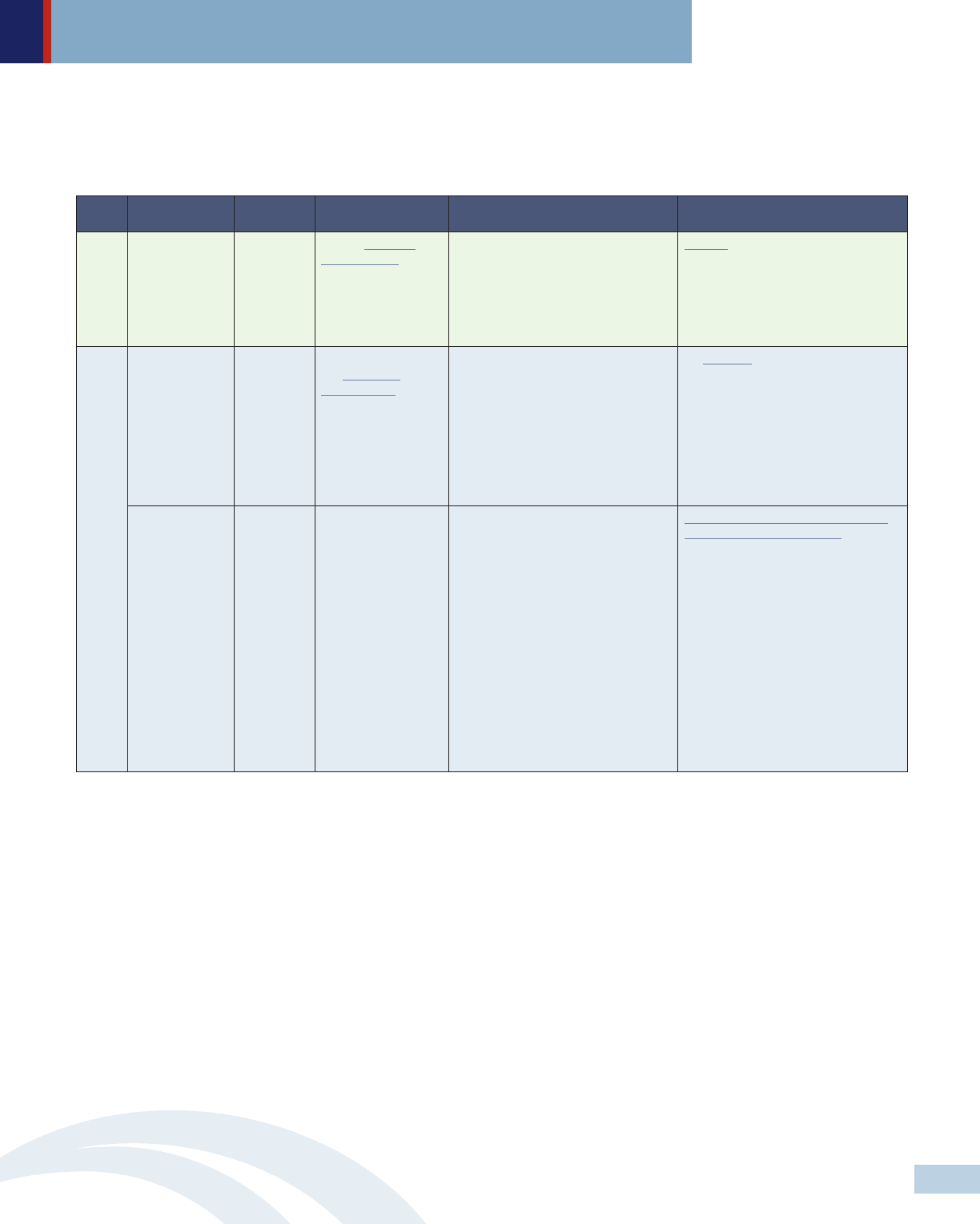
• Solution 3: Enact legislation that prohibits manipulative marketing practices that draw providers and consumers toward more
expensive alternatives. In addition, public funding should be available for academic detailing programs and consumer
education about new treatment options including their indications, contraindications and prices.
Level Policymakers Approach Policy strategies Specific recommendations Selected examples
Congress Legislation Amend Revenue
Code of 1986 to
eliminate tax
deduction for
advertising and
promotional expenses
for prescription drugs
• Ban DTCA or eliminate tax
deduction for DTCA.
S.2623 - Protecting Americans from
Drug Marketing Act 114th Congress
(2015-2016) would amend the
Internal Revenue Code to eliminate
the tax breaks that drug makers can
take to offset their spending on
prescription drug ad campaigns.
Legislature Legislation
Enforce and expand
the Physician
Sunshine Act
• Limit or ban drug manufacturers
from offering gifts to physicians.
VT prohibits manufacturers from
offering gifts, including “any payment,
food, entertainment, travel,
subscription, advance or service,” to
health care professionals, other
providers and Green Mountain Care
Board.
Legislature Legislation Establish public
funding for evidence-
based academic
detailing programs
• Provide guidance on potential
benefits and possible harms of
specific drugs.
Academic detailing programs in PA,
MA. NY, VT, ME, SC and DC have
shown to be the most effective means
to improve physician practices and
patient outcomes. Economic analysis
has also shown that they are cost-
effective.
FederalState
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
9
Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
• Solution 4: Enact legislation that aims to reduce cost-sharing and prohibit discriminatory formulary designs to ensure equitable
access to affordable prescription drugs.
Level Policymakers Approach Policy strategies Specific recommendations Selected examples
Legislature Legislation Enact legislation to
reduce cost-sharing
for prescription drugs
• Prohibit the use of coinsurance;
• Cap monthly copayment at no more
than $150 per prescription drug;
and limit the total monthly
out-of-pocket spending for
prescription drugs at a specific
threshold or perhaps at no greater
than 1/12 of the annual out-of-
pocket maximum; and
• Limit cost-sharing on prescription
drugs for people with income at or
below 150% of FPL.
DE, LA and MD set the monthly limit
copayment at $150 per specialty
drug.
CA caps out-of-pocket prescription
drug costs at no more than $250 for a
30-day prescription for most coverage.
However, this copay cap will sunset at
the end of 2019 unless legislation is
enacted to make it permanent.
Legislature
DOI
Legislation
Regulation
Enact legislation and/
or regulations that
prohibit
discriminatory
formulary design
(adverse tiering)
• Reduce the number of drug tiers
through standardized plans;
• Prohibit insurers from placing all or
most drugs that threat a specific
condition in a specialty tier; and
• Work closely with various
stakeholders including ombudsmen,
providers and consumer health
advocacy groups to identify specific
examples of discriminatory design
and put into place policies that
prohibit insurers from using these
practices.
DE prohibits insurers from placing all
drugs in a given class on a specialty
tier.
CA and CO prohibit plans from
designing formularies in the way that
discourages enrollment of individuals
with health conditions.
FL creates a drug-specific chronic
conditions template to help identify
adverse tiering—that requires plans to
identify the number, name and tier of
covered drugs used to treat certain
conditions.
State
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
10

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Introduction
The current practice of granting patent monopolies to pharmaceutical companies to spur
innovation is arguably a flawed approach to advancing continuous innovation of safe,
effective and affordable medicines.
1
Even in the context of this flawed paradigm, the
United States (U.S.) lags far behind other countries at making prescription drugs
affordable for its population. Prescription drug prices and spending are consistently
much higher in the U.S. than in other high-income countries.
2
In 2016, national expenditures on prescription drugs reached $328.6 billion, or 10.3
percent of overall national health expenditures.
3
A recent study found that the price for
four medications (Crestor, Lantus, Advair, and Humira) used to treat common conditions
is nearly three times higher in the U.S. than in other high-income countries.
4
The Center
for Medicare and Medicaid Services (CMS) projected that among the major categories of
health spending, prescription drugs would experience the fastest average growth of 6.3
percent per year over the next decade.
5
Moreover, while population growth and greater
use of prescription drugs among all age groups contribute to the rise in prescription drug
spending, a shift in prescribing toward higher price products and price increases
(especially for specialty medications) are the main factors driving average increases in
drug prices.
6
Federal policymakers are in a better position than those at the state level to drive down
drug prices as existing laws on patent rights and market exclusivity protections rest at the
federal level. However, it is unclear whether Congress and the current administration are
willing to tackle the problem in an effective way at any time soon. Therefore, to make
progress at improving prescription drug affordability, states need to lead the way.
This brief seeks to provide a policy framework to support consumer advocates in their
efforts to make prescription drugs more accessible and affordable for their state residents.
It begins with an overview of key factors contributing to the high costs of prescription
drugs in the U.S., followed by policy strategies that the federal government and states
can and should take to lower drug costs. We hope our recommendations are valuable to
consumer advocates and other stakeholders with a shared interest in taking practical
steps to ensure equitable access to affordable and effective medicines for all. While this
work requires challenging a strong pharmaceutical lobby, effective advocacy strategies
and strong grassroots support can put victories within reach.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
11

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Insurers have responded to increasing drug prices by imposing coinsurance and high
copayments and restricting access. Average out-of-pocket spending on prescription drugs
has decreased as a result of the implementation of the Affordable Care Act (ACA).
8
However, people with chronic conditions (such as cancer, digestive disease or mental
illness) are likely to spend in excess of $1,000 or more in 2014 despite having insurance
coverage.
9
While the rise in copayments and coinsurance reflect rather than cause an
increase in the underlying price of prescription drugs, they directly contribute to lack of
affordability for consumers. In a recent survey, the Kaiser Family Foundation found that
one in four of those taking a prescription drug skipped doses or cut pills in half due to
costs.
10
For millions of Americans with chronic conditions, access to effective medications
has been a persistent problem. For instance, one-third of Medicare patients with leukemia
failed to fill prescriptions within six months of diagnosis when the cost of the life-saving
drug, Gleevec, went up to $146,000 a year.
11
The effects of high prescription drug costs are not limited to the health of individual
patients. Not taking needed medications can lead to increased costs to the health care
system in the form of unnecessary hospitalizations, emergency services and physician
visits.
12
Escalating drug prices are also straining state budgets. Between 2013 and
2014, Medicaid prescription drug spending rose more than 24 percent.
13
This large
increase in spending creates a challenge for policymakers. With few tools for addressing
spending growth, a number of states have taken harmful measures such as cutting
prescription drug benefits, imposing prescription drug copays and curtailing the use of
new medicines that many people depend on.
14
Impact of Rapidly Increasing Prescription Drug Costs on
Consumers and the Government
High drug prices are a growing concern for many Americans. A majority of Americans
across the political spectrum find that prescription drug costs are unreasonable, and
want “lowering the cost of prescription drugs” to be the “top health care priority” for the
Trump administration and Congress.
7
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
12
Lowering the Cost of Prescription Drugs is
One of the Top Health Care Priorites Across Parties
Percent who say each of the following things President Trump and Congress
might do when it comes to health care is a top priority:
SOURCE: Kaiser Family Foundation Health Tracking Poll (conducted April 17-23, 2017)
DEMOCRATS INDEPENDENTS REPUBLICANS
Repealing the 2010 health care law
13% 27% 61%
Decreasing how much the federal
government spends on health care over time
22% 27% 43%
Decreasing the role of the federal
government in health care
19% 32% 51%
Dealing with the prescription
painkiller addiction epidemic
53% 46% 52%
Lowering the cost of
prescription drugs
64% 58% 60%
Lowering the amount
individuals pay for helth care
68% 56% 67%

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Key Factors Contributing to Rapidly Increasing Prescription
Drug Costs and the Lack of Affordability for Consumers
Broadly speaking, there are four key factors contributing to the high cost of prescription
drugs and the lack of affordability for consumers: (1) the monopoly power of pharmaceutical
manufacturers over drug pricing; (2) the opaque pharmaceutical supply chain that allows
various intermediary players to maximize their profits; (3) manipulative pharmaceutical
marketing campaigns affecting consumers and providers’ decisions; and (4) insurers shifting
costs to consumers by imposing high copayments and coinsurance in response to growing
prescription drug prices. This section discusses each of these factors in more detail.
• Problem 1: Pharmaceutical Monopoly Power Over Drug Pricing
The fundamental cause of high prescription drug prices in the U.S. is the failure
to counterbalance the monopoly power of pharmaceutical manufacturers with a
strong coordinated purchasing strategy. This monopoly power is conferred via
federal patent laws as well as rules that diminish federal and state authority to
negotiate drug prices or implement measures to lower drug costs.
Patent and market exclusivity rights
Various federal patent laws, including the Drug Price Competition and Patent
Term Restoration Act (commonly known as the Hatch-Waxman Act), the
Orphan Drug Act, the Biologics Price Competition and Innovation Act, and the
Generating Antibiotic Incentives Now Act give pharmaceutical manufacturers
patents and market exclusivity rights as incentives for research and development
of innovative products. Depending on the drug type, market exclusivity
15
periods vary between five to 20 years. During the period of multiyear market
protection, manufacturers of patented drugs are free to set market entry
prices—often at high levels—and annually increase drug prices.
16
At the same
time, pharmaceutical manufacturers are often able to leverage federal funds
for drug development as well as testing, marketing and commercialization.
The combination of federal funding and the absence of generic competition
enable brand-name drug manufacturers to develop and sell new drugs, recoup
their development costs and gain a high return on investment. According to
the U.S. Government Accountability Office, the pharmaceutical industry was
the most profitable industry in 2015 with an average profit margin of 17.1
percent.
17
Anticompetitive practices
Once a drug reaches the end of its exclusivity period, a generic version of the
drug is allowed to enter the market, usually at a much lower price than the
branded price. Typically, the price reduction is about 55 percent of the original
brand name cost if there are two generics on the market, 33 percent with five
generics, and 13 percent with 15 generics.
18
As a result, generic drugs quickly
capture the majority of the sales in the market formerly dominated by the
brand-name pharmaceuticals.
19
Facing a significant loss of revenue, many
brand-name companies engage in anticompetitive practices (such as pay-for
delay, product-hopping, sham citizen petitions, authorized generics and
denying access to testing samples) to limit the effect of generic competition
on drugs for which the patent is expiring.
20
These anticompetitive practices
cause substantial harm to consumers as they prevent affordable medications
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
13
Source: The Leonard D. Schaeffer
Center for Health Policy and
Economics (2017). The Flow of
Money through the Pharmaceutical
distribution System
Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
from entering the market. According to the most recent available data released
by the Federal Trade Commission (FTC), Americans pay $3.5 billion more for
prescription drugs each year because of pay-for-delay deals between brand-
name drug manufacturers and patent challengers.
21
Lack of federal and state authority to negotiate lower drug prices
Although federal and state governments, through Medicare and Medicaid, are
the largest purchasers of prescription drugs in the United States, they are
limited in their ability to negotiate for lower prices. For example, the Medicare
Prescription Drug, Improvement, and Modernization Act of 2003 includes a
‘noninterference’ provision that explicitly prohibits the Secretary of the U.S.
Department of Human and Health Services (HHS) from involvement in price
negotiations with pharmaceutical manufacturers.
• Problem 2: The Opaque Pharmaceutical Supply Chain
Over time, the supply chain for retail drugs has become increasingly complex and
lacking in transparency. Prescription drugs flow from manufacturers to various
intermediaries (including wholesalers, pharmacy benefit managers, retailers, and
private and public health insurance entities) before reaching patients. The complex
web of financial arrangements between these players creates opportunities for
profit taking at each transaction point.
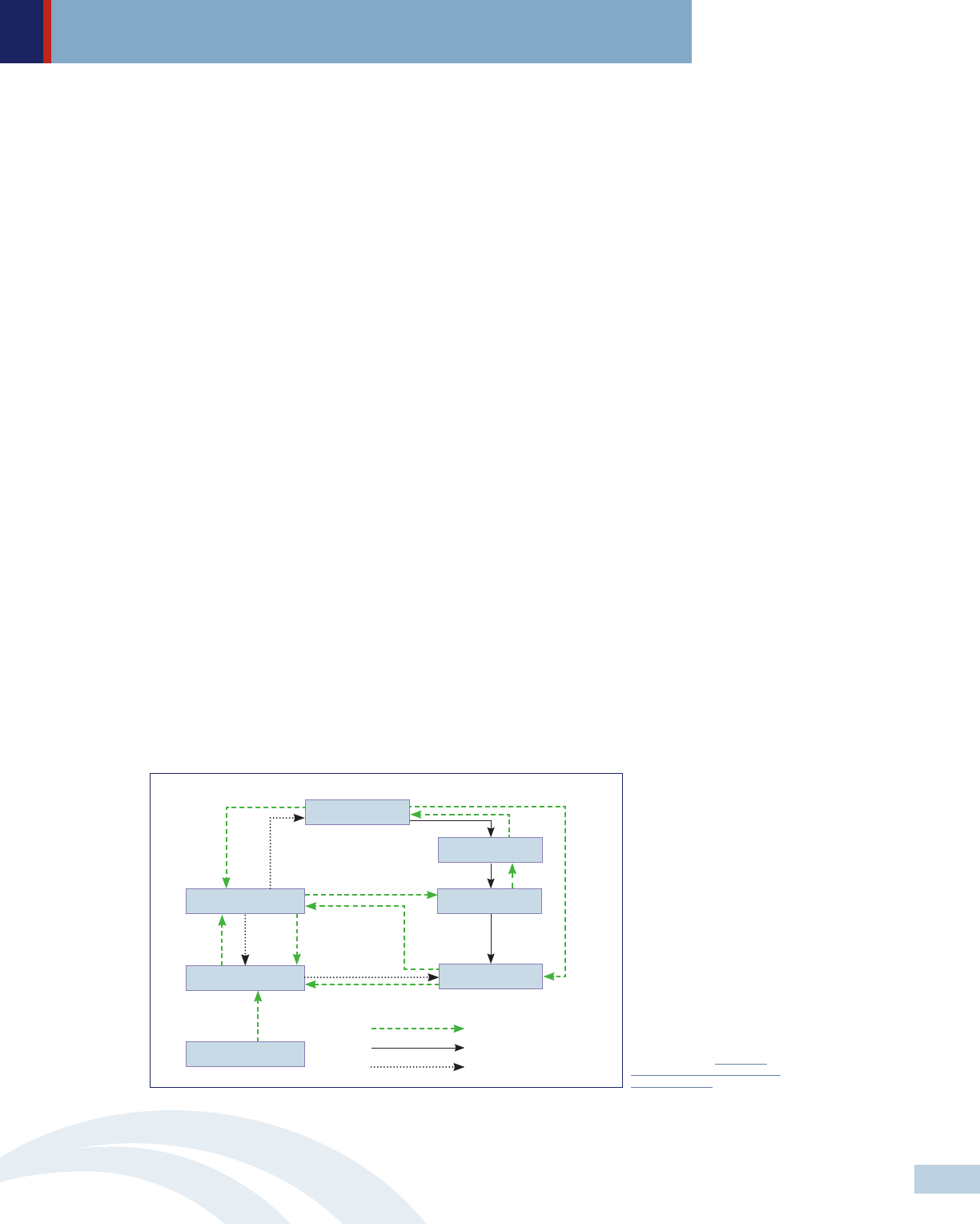
Lack of transparency and granularity of information on drug pricing decisions
Because financial arrangements among players within the pharmaceutical
distribution system often occur privately with no public records, it is difficult
to determine how large these payments are and how they are distributed. A
study conducted by the Leonard D. Schaeffer Center for Health Policy and
Economics found that consumers enrolled in high deductible health plans
sometimes pay more for a prescription drug than the insurer’s cost of acquiring
the drug.
22
While insurers acquire drugs at discount prices, consumers who
have not used up their deductible have to pay the full average wholesale price
until they reach their deductible.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
14
Formulary
payments,
market share
payments
performance
incentives,
rebates
Payment
Premium
Premium
Share of rebates
from manufacturer
Negotiated
payment
Drugs
Payment
Payment
Copay
assistance
Copay
Drugs
Drugs
Rx Drug
coverage
Managed
drug
benefits
Preferred
placement
on
formulary
Pharmacy Benefit Manager
Manufacturer
Wholesaler
Pharmacy
Beneficiary
Health Plan
Plan Sponsors
Flow of funds
Flow of Rx drugs
Services

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Pharmacy benefit manager game-playing
Pharmacy benefit managers (PBMs), the third party administrators of prescription
drug programs for private and public health insurance plans, are responsible
for developing and maintaining the formulary, contracting with pharmacies,
negotiating discounts and rebates with drug manufacturers, and processing
and paying prescription drug claims. In the past few years, some critics have
accused PBMs of driving up drug prices and interfering with patients’ access
to medications. Because some PBMs receive greater returns based on the size
of the discounts achieved, they may have little incentive to oppose high market
entry prices.
23
In addition, they may restrict access to drugs based on the
rebates they receive rather than clinical efficacy or overall cost.
24
• Problem 3: Manipulative Marketing Tactics
Drug manufacturers are spending far more on marketing than research. According
to a 2012 published on BMJ, for every dollar on “basic research,” pharmaceutical
companies invested $19 toward marketing and promotion.
25
In 2015, nearly two
thirds of the top 100 pharmaceutical manufacturers by sales spent twice as much
on marketing and sales than on research and development.
26
Pharmaceutical
manufacturers focused much of their marketing expenses on targeting physicians
to influence their prescribing practices. For instance, the industry spent more than
$3 billion on advertising to consumers, and at least $24 billion on promoting
drugs to health care professionals.
27
Tactics used include but are not limited to:
• Detailing, where pharmaceutical representatives visit doctors to pitch their
products, take them out for meals or give them gifts or free medication samples.
A new research published in JAMA found that doctors who received free meals
and other kinds of payments from pharmaceutical companies tended to prescribe
more opioid painkillers to their patients over the course of a year those who did
not get such freebies.
28
• Direct-to-consumer advertising (DTCA), which has increased nearly fourfold
since the 1997 Food and Drug Administration guidance allowing the DTCA
expansion into broadcast and electronic media.
29
The U.S. and New Zealand are
the only countries in which drug manufacturers can advertise prescription drugs
directly to consumers. Common DTCA tactics include: providing financial
assistance (e.g. copay coupons) to patients, promoting prescription products on
television, radio, print (magazines, newspapers), the Internet, and other forms
of mass media (billboards and direct mailings). Research shows that providing
copay coupons effectively steers patients away from lower-cost generic
alternatives.
30
In addition, patients are more likely to speak to their doctors
about a brand-name drug if it had been promoted on television.
31
• Grants to disease-specific patient advocacy groups, which drug manufacturers
see as allies to help build demand for new treatments and facilitate the U.S.
Food and Drug Administration (FDA) approval of experimental therapies.
According to a study published in the New England Journal of Medicine, more
than 80 percent of patient-advocacy groups accept donations from drug and
medical-device companies. For some groups, these donations accounted for
more than half of their annual revenue; and nearly 40 percent of these groups
have industry executives that sit on their governing board.
32
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
15

What Does the Affordable Care Act Say about Hospital Bills?
Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
• Problem 4: Insurers Shifting Costs To Consumers
Prescription drug affordability is important, especially for millions of Americans
living with chronic conditions, many of whom rely on more than one costly
medications. However, insurers have responded to high drug prices in at least two
ways that exacerbate affordability problems for consumers: (1) imposing high
cost-sharing through deductibles, coinsurance or copayments; or (2) adopting
discriminatory formulary designs (also known as adverse tiering) where high-cost
medications are placed in the most expensive drug tier to deter sick people from
enrolling.
High out-of-pocket cost sharing
To discourage enrollees from using high-cost drugs, many health plans assign
different levels of cost sharing to as many as four different categories of
prescription drugs: generic drugs (tier 1), preferred-brand drugs (tier 2), non-
preferred-brand drugs (tier 3), and specialty drugs (tier 4). Health plans
generally consider specialty drugs medications used to treat complex
conditions, and they are the most expensive among these four tiers. However,
the term specialty drug lacks a precise definition and drugs are sometimes
assigned to the specialty tier just based on price. The out-of-pocket cost for
medications to treat conditions like cancer, multiple sclerosis, hepatitis C or
rheumatoid arthritis, if excluded from prescription drug plans, can reach more
than $50,000 a year.
33
More than half of commercial health plans require
enrollees to pay coinsurance rather than copayments.
34
Because coinsurance
costs are based on a percentage of the drug’s price, they can be far more costly
than copays and vary significantly over time, which makes it challenging for
consumers to budget for their treatments.
35
Discriminatory formulary designs (adverse tiering)
Despite significant consumer protections under the ACA,
36
there is evidence
insurers are resorting to other tactics to dissuade high-cost patients from
enrolling, and these tactics increase prescription drug costs for people with
chronic conditions. For instance, health plans have placed most or all drugs
that treat a specific condition on the highest cost-sharing tiers and have
refused to cover commonly prescribed treatments (such as single-tablet drug
regimens).
37
Avalere Health found that some marketplace health plans place
all drugs used to treat complex diseases (such as HIV, cancer, and multiple
sclerosis) on the highest drug formulary cost-sharing tier—known as the
‘specialty’ tier.
38
As a consequence, regardless of which drugs they take,
people living with those high-cost chronic conditions enrolling in those plans
will incur significant out-of-pocket costs. A 2015 study found that consumers
living with HIV enrolling in plans with discriminatory designs had an average
annual cost per drug of $4,892, compared to $1,615 for enrollees in other
plans, and that the disparity persisted even for patients taking generic
medications.
39
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
16

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
A Policy Framework for Federal and State Actions
As a result of public demand for affordable medicines, policymakers have started turning
their attention to finding solutions that help lower prescription drug costs.
Members of Congress have filed a number of bills. Among those, the Improving Access
to Affordable Prescription Drugs Act (S.771) is the most comprehensive proposal
designed to lower drug costs while increasing innovation and promoting transparency.
40
CMS has also taken some action, proposing to allow Medicare beneficiaries to pay
coinsurance based on the discounted drug prices paid by insurers rather than the higher
retail prices.
41
Recently, the Trump administration released a blueprint for lowering high
prescription drug prices in the U.S. While the plan identifies some of the factors causing
skyrocketing costs — high list prices, a lack of negotiation tools for federal programs, and
rising out-of-pocket costs for consumers — the fundamental factor contributing to sky-
high prescription prices, the pharmaceutical monopoly power over drug pricing, is not
addressed.
42
While there are modest steps in the right direction, the Trump administration’s
action plan leaves drug corporation’s monopoly power largely untouched.
At the state level, in 2017 state policymakers introduced at least 80 bills focusing on
price transparency, unfair price increase and lowering out-of-pocket cost sharing on
prescription drugs for people with chronic conditions.
43
Despite strong pushback from
the pharmaceutical industry, five states—California, Maryland, Nevada, New York and
Oregon—have enacted groundbreaking legislation that targets excessive drug pricing. In
addition, at least eight states—California, Colorado, Delaware, Louisiana, Maryland,
Montana, New York and Vermont—have leveraged their authority to regulate health
insurance to lower prescription drug cost-sharing.
44
This year also started out strong:
within the first three weeks of 2018, lawmakers in 20 states introduced 43 bills designed
to rein in prescription drug costs.
45
Addressing prescription drug costs requires actions at both the federal and state level. We
use the following policy framework to address each of the four problems contributing to
the fast-growing drug costs discussed above. Specifically, we recommend policymakers to:
• Solution 1: Enact Legislation And Leverage Existing Federal Authorities To
Reduce Pharmaceutical Monopoly Power Over Drug Pricing
At the federal level, Congress should:
- Leverage existing laws, such as ‘March-In Rights’ (35 U.S.C. §203) and ‘Patent
& Copyright’ (28 U.S.C. §1498), to force down prescription drug prices. For
instance, in case of supply shortage or exorbitant price hikes, HHS has the right
under 35 U.S.C. §203 to force patent manufacturers that used taxpayers’ dollars
to develop their innovations to allow drugs to enter the market at cheaper prices.
HHS can also invoke the government use of patented interventions under 28
U.S.C. §1498 to license generic version of high-cost medications at low prices.
46
This approach was used in the 1950s and 1960s to procure cheaper drugs.
47
Early this year, the State of Louisiana Department of Health was considering
using 28 U.S.C. §1498 to bypass Gilead’s patents on Sovaldi and Harvoni, two
of the new, highly effective hepatitis C drugs, to treat people with hepatitis C in
the state’s Medicaid program, prison system, and its uninsured.
48
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
17

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
- Amend the Hatch-Waxman Act, Orphan Drug Act, the Biologics Price Competition
and Innovation Act and the Generating Antibiotic Incentives Now Act to:
49
(1) Shorten patent and market exclusivity periods and eliminate patent
extensions. For instance, the U.S. is the only country that allows a 12-year
exclusivity period for biologics, which discourages the development of
biosimilars. Experts suggest it would make more sense to grant brand-
name drug manufacturers only up to seven years of market exclusivity for
biologics.
50
In addition, to rebalance innovation incentives and competition,
Congress should eliminate patent extensions for drugs that have no
demonstrated added value compared to those already on the market.
(2) Amend ‘March-In Rights’ (35 U.S.C. §203) to set limits on introductory
prices for new innovative drugs and annual price increases for existing
drugs that receive federal funding for research and development. Under 35
U.S.C. §203, the federal government has the authority to “march-in” in
the in the event that high price is preventing a drug developed with federal
funding for research and development from being available or affordable.
The proposed amendment is to allow federal government to prospectively
review the launch price of a drug developed with federal support.
(3) Prohibit anti-competitive practices (such as pay-for-delay, product-hopping,
sham citizen petitions, and authorized generics) that lead to high drug
prices. Congress should increase FTC resources to monitor, oversee and
investigate drug manufacturers engaging in anticompetitive practices. In
addition, the FDA should be empowered to terminate market exclusivity on
any product found to be in violation. All patent claims (including biologics)
should be disclosed in the Orange Book at the time of originator drug
registration—not years later when the originator is trying to block the
generic manufacturers from market entry.
- Remove the ‘non-interference’ clause in the Medicare Prescription Drug,
Improvement, and Modernization Act of 2003, so Medicare could directly
negotiate Part D drug prices with pharmaceutical manufacturers. According to a
2007 analysis conducted by the Congressional Budget Office, savings for
Medicare could occur if the HHS Secretary has the authority to negotiate lower
prices for a broad set of drugs or drug types (including many of today’s high-
priced specialty drugs and biologics) on behalf of Medicare beneficiaries.
51
In
addition, Congress should allow HHS to limit what Medicare pays for a drug
based on the price of another therapeutically equivalent drug.
At the state level, states should:
- Establish multistate and/or in-state collaborative
(1) Establish multistate purchasing pools to negotiate reduced prices of high
cost medications. States could seek state plan amendment approvals to
establish collaborations among states to purchase large quantities of high-
cost medications in exchange for favorable rebates. This strategy can limit
annual price increases, eliminate differences in costs between participating
states and ensure access to critical medications for low-income people.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
18
Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Currently, there are four multistate purchasing pools in which more than
half of the states are participating.
52
In addition to the federally required
rebate, which is approximately 50 percent of initial payments, drug
manufacturers offer these states supplemental rebates.
(2) Operate as PBMs to broaden purchasing and negotiating power. Similar to
multistate purchasing pools, states can pool in-state participants and use
unified formularies for all covered members across state and local programs.
- Enact legislation that requires pharmaceutical manufacturers to (1) justify
excessive price increases for both generic and brand name drugs or face
penalties; or (2) provide rebates when prices exceed a specific threshold.
53
Maryland passed into law price-gouging legislation, HB 631, which authorizes
the state attorney general to take legal action to stop drug manufacturers from
engaging in unconscionable price increases in noncompetitive off-patent or
generic drug markets. Despite limitations within HB 631, which is focusing only
on generic drugs and is lacking public disclosure of information collected by the
state attorney general, Maryland’s law presents a new tool to hold drug
manufacturers accountable and deter price hikes in the state.
54
A three judge
panel of the U.S. Court of Appeals for the Fourth Circuit recently ruled 2-1 that
Maryland’s anti-price gouging law unconstitutionally violates the “Dormant
Commerce Clause,” because, in their view it would affect transactions in other
states.
55
Maryland Attorney General Brian Frosh has appealed the ruling to the
full Fourth Circuit Court of Appeals.
56
State advocates are hopeful that the
Attorney General will prevail and get the anti-price gouging law reinstated.
Interestingly, a few days after the court decision on Maryland’s law, the Illinois
House of Representatives passed a similar legislation, HB4900, that would give
the Illinois attorney general the power to stop price gouging of essential off-
patent or generic drugs. The Illinois State Senate is expected to vote on that bill
in May.
- Create an independent committee to review price setting and annual price
increases for prescription drugs. To ensure consumers are protected from
unreasonable prescription drug costs, state agencies should have the authority
to evaluate the reasonableness of drug prices. Specifically, for a prescription
drug that triggers criteria for a review, state agencies should be able to approve
or reject a proposed price before it is allowed to be sold in the state. Maryland
introduced a drug cost review bill to evaluate the affordability for certain drugs.
The proposed bill establishes a Drug Cost Review Commission and an advisory
board to closely evaluate high cost drug prices and set the rates at which
Marylanders would pay for those drugs, based on a drug’s cost and affordability.
- Offer public input opportunities. Consumers should have opportunities to provide
input through either a comment period, a public hearing, or formal appeals
process.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
19
Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
• Solution 2: Enact Legislation That Mandates Public Disclosure On Drug Pricing,
Investment In Drug Development, Manufacturing And Marketing In Order To
Break Down The Opaque Pharmaceutical Supply Chain
The following policy strategies can be undertaken at the state level.
- Require department of health or relevant agencies to set a price cap for a specific
drug and require drug manufacturers to submit justifications for all drugs with a
price cap. The price cap could be set at a fixed percentage above the average
price for that drug sold in the country members of the Organization for Economic
Co-operation and Development (OECD). For example, Maryland has proposed to
cap the amount that the state and commercial health plans would pay for certain
drugs rather than the amount drug manufacturers would charge for them.
Specifically, the legislation, based on NASHP’s Model Drug Price Transparency
Act,
57
requires drug manufacturer to justify their price setting for certain drugs,
with an annual cost above $30,000. New York enacted legislation, S.2007B/A.
3007B, that requires the state Department of Health to establish a Medicaid
spending cap with year-to-year spending targets and to conduct drug expenditure
reviews quarterly. If expenditures exceed the department’s spending cap, the
commissioner identifies and refers specific drugs to a drug utilization board for
recommended supplemental rebates. At least 30 drugs were under review after
the law was implemented, and the state asked for supplemental rebates from 12
manufacturers—most complied.
58
- Require drug manufacturers to submit justifications for all drugs that have a
10-percent price increase above the previous price and to undergo a price review
process. Pharmaceutical manufacturers should be required to publicly disclose
detailed information on prescription drug pricing as well as development,
manufacturing and marketing costs on a drug-by-drug basis, and grants to non-
profit advocacy groups. Nevada enacted legislation, SB 539, targeting two
specific groups of drugs that are used to treat diabetes, insulin and biguanides.
Diabetes drug manufacturers are now required to disclose information about the
costs of making and marketing drugs when prices increase by a certain amount.
Nevada’s law also requires PBMs and nonprofit groups receiving grants from
pharmaceutical companies to submit annual reports to the state department of
health and human services of their financial arrangements with drug
manufacturers. Recently, Oregon enacted legislation, HB 4005, to require
pharmaceutical manufacturers to compile a report on a prescription drug if the
price was $100 or more for a one-month supply (or course of treatment lasting
less than 1 month) and if the net price increased by 10 percent or more. In
addition, Oregon’s Department of Consumer and Business Services is required to
post a list of high drug price increases. Furthermore, insurers are required to
show how these drug price increases affect premiums.
- Require pharmaceutical manufacturers to provide advance notices of price hikes
to give time for purchasers to adjust formularies, negotiate price concessions or
seek other alternatives. California passed into law a bill, SB 17, which aims to
increase transparency in prescription drug pricing through advance notice and
public information about the costs of prescription drugs. Drug manufacturers are
required to disclose information about drug pricing to the Office of Statewide
Health Planning and Development. SB 17 also requires a 60-day advance notice
of price hikes for most prescription drugs. Insurers are also required to publicly
disclose, through rate review, the percentage of the premium attributable to
prescription drug costs.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
20

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
• Solution 3: Enact Legislation That Prohibits Manipulative Marketing Practices
That Lure Providers And Consumers Toward More Expensive Alternatives
At the federal level, Congress should:
- Enact legislation that bans DTCA or eliminates the tax deduction for DTCA. It is
irresponsible to promote unnecessarily expensive drugs to consumers lacking
medical knowledge to make smart informed decisions. The American Medical
Association has called for a ban on advertising prescription drugs and medical
devices directly to consumers.
59
In 2016, a group of Democratic senators
(including Al Franken (D-Minnesota), Sheldon Whitehouse (D-Rhode Island),
Sherrod Brown (D-Ohio) and Tom Udall (D-New Mexico)) introduced the
Protecting Americans from Drug Marketing Act to amend the Internal Revenue
Code of 1986 to eliminate the tax breaks that drug makers can take to offset
their spending on ad campaigns.
60
Savings generated from the elimination of
these tax breaks should be used to fund academic detailing programs
61
and
educate consumers about how to review prescription drug ads.
At the state level, states should:
- Limit or ban physician gifts. The Physician Payment Sunshine provisions under
the ACA require drug and medical device manufacturers to publicly report gifts
and payments made to physicians and teaching hospitals. However, the law does
not limit financial relationships between these entities. Vermont’s law goes
beyond the Physician Payment Sunshine Act to prohibit manufacturers from
offering gifts, including “any payment, food, entertainment, travel, subscription,
advance or service,” to health care professionals, other providers and Green
Mountain Care Board.
62
- Establish public funding for evidence-based academic detailing programs.
Located in medical schools or schools of pharmacy, academic detailing programs
operate independently from drug manufacturers. These programs provide
prescribers with reliable guidance on potential benefits and possible harms of
specific drugs. These programs have shown to be the most effective means to
improve physician practices and patient outcomes. Several states, including
Pennsylvania, Massachusetts, New York, Vermont, Maine, South Carolina, and the
District of Columbia, have established academic detailing programs, which have
proven to be cost-effective. Studies of existing state programs found that every
$1 invested in these programs results in a $2 return on investment.
63
• Solution 4: Enact Legislation And Regulations That Aim To Reduce Cost Sharing
And Prohibit Discriminatory Formulary Designs (Known As Adverse Tiering) To
Ensure Equitable Access To Affordable Medications
States should enact legislation to:
- Reduce cost-sharing on prescription drugs by:
(1) Reducing the number of drug tiers through standardized plans that limit
the number of specialty brand name, and generic tiers. Massachusetts, New
York and Vermont have limited plans to three formulary tiers.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
21

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
(2) Prohibiting the use of coinsurance and capping monthly copayments at no
more than $150 for prescription drugs. No states have prohibited the use
of coinsurance, but at least eight states have limited monthly out-of-pocket
payments of patients in private health plans. Delaware, Louisiana and
Maryland set the monthly limit at $150 per specialty drug. California caps
out-of-pocket prescription drug costs at no more than $250 for a 30-day
supply of a prescription drug for most coverage.
64
Note that the co-pay
caps for prescription drugs in California will sunset at the end of 2019
unless the state enacts legislation to make this requirement permanent. In
addition, states should consider capping the total annual out-of-pocket
spending for prescription drugs at a specific dollar threshold or perhaps no
greater than one-twelfth of the annual out-of-pocket maximum. For
consumers who rely on multiple prescription drugs a month, this could
significantly increase improve affordability.
(3) Limit cost-sharing on prescription drugs for people with income at or below
150 percent of the federal poverty level. For people with this income level
who likely have less disposable income, any spending on health insurance
premiums or cost-sharing would come not from discretionary income, but
rather at the expense of the ability to afford food, clothing, shelter and
other necessities. Therefore, states should consider requiring only nominal
amounts of cost sharing on prescription drugs or eliminate it altogether.
- Prohibit discriminatory formulary designs (also known as adverse tiering) by:
(1) Prohibiting insurers from placing all or most drugs that treat a specific
condition in a specialty tier. Delaware prohibits insurers from placing all
drugs in a given class on a specialty tier. Similarly, California and Colorado
prohibits plans from designing formularies in the way that discourages
enrollment of individuals with health conditions. Florida creates a drug-
specific chronic conditions template—a tool to help identify adverse
tiering—that requires plans to identify the number, name and tier of
covered drugs used to treat certain conditions.
65
(2) Working closely with various stakeholders including ombudsmen, providers
and consumer health advocacy groups to identify specific examples of
discriminatory formulary design and putting into place policies that prohibit
insurers from these practices.
Conclusion
The fast-growing cost of prescription drugs has become a top health care concern for
many Americans. Unless policymakers come up with effective strategies to drive down
drug prices, many people, especially those living with chronic conditions, will continue
to struggle to pay for their medications and end up skipping doses or deciding to not fill
their prescription at all. This leads to poorer health and higher health care costs.
Consumer advocates, policymakers at both the federal and state levels, and stakeholders
can work together to adopt measures that help curb unfair drug pricing and improve
affordability for consumers. The work often seems overwhelming given the power and
resources of the pharmaceutical lobby. However, with political will, effective advocacy
strategies and strong grassroots support, victories are within reach.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
22

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Glossary
Authorized generics: are not generic but branded products sold under generic names.
Brand-name drug companies often use this tactic to compete with generic companies.
By law, the first generic company is granted an exclusivity period of 180 days to market
a new generic product. During this time, the FDA might not approve any additional
generic competitors. However, the 180-day exclusivity does not preclude a company with
the expiring patent from launching an authorized generic. This means by selling a drug
they are already making under a different name, band-name drug companies are drawing
revenue away from generic companies during the 180-day exclusivity, thus effectively
extending their monopoly for another six months.
66
The Biologics Price Competition and Innovation Act (BPCIA) (Public Law 111-148) was
signed into law as part of the Affordable Care Act in 2010 to create an approval pathway
for drugs that are highly similar (biosimilar) to or interchangeable with biological products
(drugs made from human and/or animal materials). The BPCIA establishes a 12-year
data exclusivity for new biological structures and a one-year exclusivity for biosimilars.
The law also creates a patent dispute resolution process that requires the biosimilar
sponsor to disclose information about its manufacturing process to the relevant patent
holder. A series of informational exchanges then occur in which the biosimilar sponsor
and the original patent holder identify a list of patents that are in question. The validity
of the claims of infringement can then be adjudicated.
67
Sham citizen petition: Brand-name drug companies file “sham” citizen petitions to ask
the FDA to delay action on a pending generic application. Many of these petitions are
submitted near the date of patent expiration, effectively limiting potential competition
for another 150 days. According to the FDA, brand-name drug companies submit 92
percent of all citizen petitions.
68
The Drug Price Competition and Patent Term Restoration Act (Public Law 98-417) commonly
known as the Hatch-Waxman Act, was enacted in 1984 with two main goals: (1) to grant
brand-name drug companies extensions in market exclusivity as incentives for innovation
in pharmaceutical research and development; and (2) to create price competitions by
helping low-cost and high-quality generic drugs enter the market quickly. At first, the
Hatch-Waxman Act had a positive record of success with a robust generic drug market.
By 2012, generic drugs became the standard of care for many common diseases as they
were less expensive than branded drugs and were available in nearly every therapeutic
class. However, after 30 years of success, numerous problems have emerged. Some were
the results of deliberate manipulation of the law by the pharmaceutical industry to
maximize their profits, others involve interpretation of the statute by the Supreme Court
in a way that unintentionally limits the liability of generic drug companies when patients
are harmed by their drugs, which may disincentive future uses of generic drugs.
69
The Federal Medicaid Rebate Program was created by the Ominibus Budget Reconciliation
Act of 1990 to help lower Medicaid spending on outpatient prescription drugs by ensuring
states receive discounts similar to those provided to private purchasers. Under this
program, participating drug manufacturers are required to enter into a national rebate
agreement with the HHS secretary in exchange for state Medicaid coverage of most of
their products. Approximately 600 drug manufacturers and all 50 states are in the
program. The program has generated significant revenue for the states (and the federal
government) and helped offset Medicaid prescription drug expenditures. However, states,
payers and drug manufacturers are considering whether the program is an effective
approach to lower drug costs and improve access to therapies.
70
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
23
Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
The Generating Antibiotic Incentives Now (GAIN Act) provisions (Public Law 112–144) was
signed into law in 2012 as part of the Food and Drug Administration Safety and Innovation
Act. The GAIN Act extends an additional five years of exclusivity for new antibiotics that
are qualified as infectious disease products—antibacterial or antifungal drugs to treat
serious or life-threatening infections. This extra five years of market protection is in
addition to any existing exclusivity, including that which may be applicable under Hatch-
Waxman, orphan drug or pediatric exclusivity.
71
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (Public law
108 -173) also called the Medicare Modernization Act includes the Medicare Part D
prescription drug benefit plan. Under Part D, private plans negotiate drug prices with
drug manufacturers and structure benefits (including formularies, cost-sharing, utilization
management policies and preferred pharmacies). While creating an opportunity for
Medicare beneficiaries to purchase drug coverage—a choice they had not have before,
the Medicare Modernization Act has effectively expanded the role of private plans in
Medicare and prohibited any interference by HHS with respect to drug prices.
72
The Orange Book (Approved Drug Product with Therapeutic Equivalence Evaluations).
Required by the Hatch-Waxman Act, FDA publishes the Orange Book that lists drug
products approved on the basis of safety and effectiveness under the Federal Food, Drug,
and Cosmetic Act. The Orange Book contains therapeutic equivalence evaluations for
approved multisource prescription drug products. These evaluations have been prepared
to serve as public information and advice to state health agencies, prescribers, and
pharmacists to promote public education in the area of drug product selection and to
foster containment of health care costs. Inclusion of products in the Orange Book is
independent of any current regulatory actions through administrative or judicial means
against a drug product. In addition, therapeutic equivalence evaluations in this publication
are not official FDA actions affecting the legal status of products under the Federal Food,
Drug, and Cosmetic Act.
73
The Orphan Drug Act (Public Law 97-414) was enacted in 1983 to stimulate the
development of orphan drugs—drugs for rare diseases (such as Huntington’s Disease,
myoclonus, ALS, Tourette syndrome and muscular dystrophy) that affect fewer than
200,000 Americans. Prior to its passage, the pharmaceutical industry had little incentive
to invest money in the development of treatments for small patient populations, because
the drugs were expected to be unprofitable. The law provides seven-year market exclusivity
to sponsors of approved orphan products, a tax credit of 50 percent of the cost of
conducting human clinical testing, and research grants for clinical testing of new
therapies to treat orphan diseases. These incentives have encouraged the pharmaceutical
industry to accelerate research and development of these drugs allowing patients with
orphan diseases access to treatment. However, many drugs that have gained the orphan
drug status are not entirely new. According to a Kaiser Health News investigation, more
than 70 percent of these drugs were first approved by FDA for mass market use. These
medicines were later approved as orphans.
74
Pay-for-delay: In a pay-for-delay deal, a brand-name drug company pays off a would-be
competitor to delay it from selling a generic version of the drug. Without any competition,
the brand-name company can continue demanding high prices for its drug.
75
Product-hopping: is a tactic by which brand-name drug companies can try to obstruct
generic competitors and preserve monopoly profits on a patented drug by making modest
reformulations that offer little or no therapeutic advantages. Product-hopping is also
called ever greening.
76
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
24

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Risk evaluation and mitigation strategy (REMS): In 2007, Congress enacted the Food and
Drug Administration Amendments Act (FDAAA) that require generic sponsors of drug
applications to submit a proposed REMS if the FDA determines that it is needed to
ensure a drug’s benefits outweigh its risks. Six factors considered for REMS include: (1)
the population size likely to use the drugs; (2) the seriousness of the disease; (3) the
drug’s expected benefit; (4) the expected duration of treatment; (5) the seriousness of
adverse effects; and (6) the drug’s novelty. The FDA can require a REMS before a drug
enters the market based on known risks or after the drug has been approved based on
new evidence of risk. Brand-name manufacturers have use this regulatory strategy to
block generic companies from getting testing samples.
77
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
25

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Endnotes
1
Joseph E. Stiglitz. Prizes, Not Patents. Project Syndicate, March 6, 2007. https://www.project-syndicate.
org/commentary/prizes--not-patents?barrier=accessreg. Accessed April 27, 2018.
2
D. O. Sarnak, D. Squires, G. Kuzmak, and S. Bishop, Paying for Prescription Drugs Around the World: Why
Is the U.S. an Outlier? The Commonwealth Fund, October 2017. http://www.commonwealthfund.org/
publications/issue-briefs/2017/oct/prescription-drug-costs-us-outlier. Accessed April 27, 2018.
3
Micah Hartman, Anne B. Martin, Nathan Espinosa, Aaron Catlin, and The National Health Expenditure
Accounts Team. National Health Care Spending In 2016: Spending And Enrollment Growth Slow After
Initial Coverage Expansions. Health Affairs 37, No. 1 (2018): 150–160. doi: 10.1377/
hlthaff.2017.1299. Accessed April 27, 2018.
4
Papanicolas I, Woskie LR, Jha AK. Health Care Spending in the United States and Other High-Income
Countries. JAMA. 2018; 319(10):1024–1039. doi:10.1001/jama.2018.1150. Accessed April 27, 2018.
5
Gigi A. Cuckler, Andrea M. Sisko, John A. Poisal, Sean P. Keehan, Sheila D. Smith, Andrew J. Madison,
Christian J. Wolfe, and James C. Hardesty. National Health Expenditure Projections, 2017–26: Despite
Uncertainty, Fundamentals Primarily Drive Spending Growth. Health Affairs 37, NO. 3 (2018): 482–492.
doi: 10.1377/hlthaff.2017.1655. Accessed April 27, 2018.
6
The Department of Health and Human Services. Observations on Trends in Prescription Drug Spending.
ASPE Issue Brief. March 2016. https://aspe.hhs.gov/system/files/pdf/187586/Drugspending.pdf. Accessed
April 27, 2018.
7
Henry J Kaiser Family Foundation. Public Opinion on Prescription Drugs and Their Prices: Poll Findings
from 2015-2018. KFF Health Tracking Polls. https://www.kff.org/slideshow/public-opinion-on-prescription-
drugs-and-their-prices/. Accessed April 27, 2018.
8
Andrew W. Mulcahy, Christine Eibner, and Kenneth Finegold. Gaining Coverage Through Medicaid Or
Private Insurance Increased Prescription Use And Lowered Out-Of-Pocket Spending. Health Affairs 35,
NO. 9 (2016): 1725–1733. doi: 10.1377/hlthaff.2016.0091. Accessed April 27, 2018.
9
Cynthia Cox, Anthony Damico, Gary Claxton and Larry Levitt. Examining High Prescription Drug Spending
For People With Employer Sponsored Health Insurance. Henry J Kaiser Family Foundation. October 2016.
https://www.healthsystemtracker.org/brief/examining-high-prescription-drug-spending-for-people-with-
employer-sponsored-health-insurance/#item-start. Accessed April 27, 2018.
10
Bianca DiJulio, Ashley Kirzinger, Bryan Wu, and Mollyann Brodie. Data Note: Americans’ Challenges with
Health Care Costs. Henry J Kaiser Family Foundation. March 2017. http://www.kff.org/health-costs/
poll-finding/data-note-americans-challenges-with-health-care-costs/. Accessed April 27, 2018.
11
Liz Szabo. As Drug Costs Soar, People Delay Or Skip Cancer Treatments. NPR Shots. March 15, 2017.
http://www.npr.org/sections/health-shots/2017/03/15/520110742/as-drug-costs-soar-people-delay-or-skip-
cancer-treatments. Accessed April 27, 2018.
12
Jane E. Brody. The Cost of Not Taking Your Medicine. The New York Times. April 17, 2017. https://www.
nytimes.com/2017/04/17/well/the-cost-of-not-taking-your-medicine.html?_r=0. Accessed April 27, 2018.
13
Medicaid and CHIP Payment and Access Commission (MACPAC). Medicaid Spending for Prescription
Drugs. MACPAC. January 2016. https://www.macpac.gov/wp-content/uploads/2016/01/Medicaid-
Spending-for-Prescription-Drugs.pdf. Accessed April 27, 2018.
14
The Kaiser Family Foundation. State Health Facts: Medicaid Benefits: Prescription Drugs. 2012. https://
www.kff.org/medicaid/state-indicator/prescription-drugs/. Accessed April 27, 2018.
15
The term “market exclusivity” can be confusing as there are three forms of extended protection for
monopoly pricing: (1) patents; (2) data exclusivity where one can still submit independently clinical trial
data, just not rely on data from already registered originator drug; and (3) market exclusivity where one is
precluded from submitting for registration of a follow-on product.
16
Naren P. Tallapragada. Off-Patent Drugs At Brand-Name Prices: A Puzzle For Policymakers. Journal of Law
and the Biosciences. 2016; 3(1):238-247. doi:10.1093/jlb/lsw008. Accessed April 27, 2018.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
26

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
17
U.S. Government Accountability Office. Drug Industry: Profits, Research and Development Spending, and
Merger and Acquisition Deals. November 2017. https://www.gao.gov/assets/690/688472.pdf. Accessed
April 27, 2018.
18
Aaron S. Kesselheim, MD, JD, MPH; Jerry Avorn, MD; Ameet Sarpatwari, JD, PhD. The High Cost of
Prescription Drugs in the United States Origins and Prospects for Reform. JAMA. 2016; 316(8): 858-
871. doi: 10.1001/jama.2016.11237. Accessed April 27, 2018.
19
David Balto. Removing Obstacles to Generic Drug Competition: A Critical Priority for Health Care Reform.
Center for American Progress. June 2009. https://www.americanprogress.org/issues/economy/
reports/2009/06/23/6281/removing-obstacles-to-generic-drug-competition/. Accessed April 27, 2018.
20
Cynthia Koons and Robert Langreth. The Loopholes Drug Companies Use to Keep Prices High. Bloomberg.
December 20, 2017. https://www.bloomberg.com/news/features/2017-12-20/the-loopholes-drug-
companies-use-to-keep-prices-high. Accessed April 27, 2018.
21
Federal Trade Commission. Pay-for-Delay: How Drug Company Pay-Offs Cost Consumers Billions. 2010.
https://www.ftc.gov/sites/default/files/documents/reports/pay-delay-how-drug-company-pay-offs-cost-
consumers-billions-federal-trade-commission-staff-study/100112payfordelayrpt.pdf. Accessed April 27,
2018.
22
Neeraj Sood, PhD; Tiffany Shih, PhD; Karen Van Nuys, PhD; Dana Goldman, PhD. The Flow of Money
through the Pharmaceutical distribution System. The Leonard D. Schaeffer Center for Health Policy and
Economics. June 2017. http://healthpolicy.usc.edu/documents/USC%20Schaeffer_Flow%20of%20
Money_2017.pdf. Accessed April 27, 2018.
23
Kevin A. Schulman, MD; and Barak D. Richman, JD, PhD. The Evolving Pharmaceutical Benefits Market.
JAMA. April 2018. doi:10.1001/jama.2018.4269. Accessed April 27, 2018.
24
David Dayen. The Hidden Monopolies That Raise Drug Prices: How Pharmacy Benefit Managers Morphed
From Processors To Predators. The American Prospect, March 28, 2018. http://prospect.org/article/
hidden-monopolies-raise-drug-prices. Accessed April 27, 2018.
25
Donald W Light, professor 1, Joel R Lexchin. Pharmaceutical Research And Development: What Do We
Get For All That Money? BMJ 2012; 345:e4348. https://doi.org/10.1136/bmj.e4348 Accessed April 27,
2018.
26
Institute for Health and Socio-Economic Policy. The R&D Smokescreen: The Prioritization of Marketing &
Sales in the Pharmaceutical Industry. October 2016. http://nurses.3cdn.net/e74ab9a3e937fe5646_
afm6bh0u9.pdf. Accessed April 27, 2018.
27
The Pew Charitable Trust. Persuading the Prescribers: Pharmaceutical Industry Marketing and its
Influence on Physicians and Patients. November 2013. http://www.pewtrusts.org/en/research-and-analysis/
fact-sheets/2013/11/11/persuading-the-prescribers-pharmaceutical-industry-marketing-and-its-influence-
on-physicians-and-patients. Accessed April 27, 2018.
28
Scott E. Hadland, MD, MPH, MS; Magdalena Cerdá, DrPH, MPH; Yu Li, MD, PhD; et al Maxwell S.
Krieger, BS; Brandon D. L. Marshall, PhD. Association of Pharmaceutical Industry Marketing of Opioid
Products to Physicians With Subsequent Opioid Prescribing. JAMA. May 2018. doi:10.1001/
jamainternmed.2018.1999. Accessed May 21, 2018.
29
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug
Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for
Veterinary Medicine (CVM). Guidance for Industry Consumer-Directed Broadcast, Advertisements,
Questions and Answers. August 1999. https://www.fda.gov/downloads/Drugs/
GuidanceComplianceRegulatoryInformation/Guidances/UCM122825.pdf. Accessed April 27, 2018.
30
Leemore Dafny, Christopher Ody, Matt Schmitt. When Discounts Raise Costs: The Effect of Copay Coupons
on Generic Utilization. UCLA Anderson School of Management, October 4, 2016. http://www.hbs.edu/
faculty/Publication%20Files/DafnyOdySchmitt_CopayCoupons_32601e45-849b-4280-9992-
2c3e03bc8cc4.pdf. Accessed April 27, 2018.
31
John Henning Schumann. Those TV Drug Ads Distract Us From The Medical Care We Need. NPR Shots,
April 29, 2017. https://www.npr.org/sections/health-shots/2017/04/29/525877472/those-tv-drug-ads-
distract-us-from-the-medical-care-we-need. Accessed April 27, 2018.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
27

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
32
Matthew S. McCoy, Ph.D., Michael Carniol, M.B.A., Katherine Chockley, B.A., John W. Urwin, B.S.,
Ezekiel J. Emanuel, M.D., Ph.D., and Harald Schmidt, Ph.D. Conflict of Interest for Patient-Advocacy
Groups. The New England Journal of Medicine 2017; 376: 880-885. http://www.nejm.org/doi/
pdf/10.1056/NEJMsr1610625. Accessed April 27, 2018.
33
Alisan Kodjak. Specialty Drugs Can Prove Expensive Even With Medicare Coverage. NPR Shots, December
3, 2015. https://www.npr.org/sections/health-shots/2015/12/03/458216778/specialty-drugs-can-prove-
expensive-even-with-medicare-coverage. Accessed April 27, 2018.
34
Sandy Ahn and Sabrina Corlette. State Efforts to Lower Consumer Cost-Sharing for High-Cost Prescription
Drugs. Urban Institute. August 2017. https://www.urban.org/sites/default/files/
publication/93071/2001491-state-efforts-lower-rx-cost-sharing.pdf. Accessed April 27, 2018.
35
Ibid. footnote 32.
36
A number of consumer protections under the ACA include: guaranteed issue, bans on preexisting condition
exclusions and lifetime/annual limits, new rating reforms, essential health benefits and prohibiting
discrimination based on a variety of factors including health status. However, these protections could be
undercut if short-term health insurance that don’t meet the ACA requirement become more widely
available.
37
Michelle Andrews. 7 Insurers Alleged To Have Discriminated Against HIV Patients. NPR Shots, October
18, 2016. http://www.npr.org/sections/health-shots/2016/10/18/498427561/7-insurers-alleged-to-have-
discrimated-against-hiv-patients. Accessed April 27, 2018.
38
Avalere PlanScape
®
. Prescription Drug Tier Placement and Cost Sharing in Health Insurance Exchange
Plans. Avalere. February 2015.http://go.avalere.com/acton/attachment/12909/f-017c/1/-/-/-/-/20150211_
Avalere%20Planscape%202015_Class%20Tiering%20Analysis.pdf. Accessed April 27, 2018.
39
Douglas B. Jacobs, Sc.B., and Benjamin D. Sommers, M.D., Ph.D. Using Drugs to Discriminate —
Adverse Selection in the Insurance Marketplace. The New England Journal of Medicine 2015; 372:399-
402. doi: 10.1056/NEJMp1411376. Accessed April 27, 2018.
40
S.771 - Improving Access To Affordable Prescription Drugs Act. 115th Congress (2017-2018). https://
www.congress.gov/bill/115th-congress/senate-bill/771. Accessed April 27, 2018.
41
CMS-4182-P: Medicare Program; Contract Year 2019 Policy and Technical Changes to the Medicare
Advantage, Medicare Cost Plan, Medicare Fee-for-Service, the Medicare Prescription Drug Benefit
Programs, and the PACE Program (Proposed Rule). https://s3.amazonaws.com/public-inspection.
federalregister.gov/2017-25068.pdf. Accessed April 27, 2018.
42
Department of Health and Human Services U.S.A. American Patients First: The Trump Administration
Blueprint to Lower Drug Prices and Reduce Out-Of-Pocket Costs. May 2018. https://www.hhs.gov/sites/
default/files/AmericanPatientsFirst.pdf Accessed May 21, 2018.)
43
Aaron Berman, Theodore Lee, Adam Pan, Zain Rizvi, Arielle Thomas. Curbing Unfair Drug Prices: A Primer
for States. The Yale Global Health Justice Partnership. August 2017. https://law.yale.edu/system/files/area/
center/ghjp/documents/curbing_unfair_drug_prices-policy_paper-080717.pdf. Accessed April 27, 2018.
44
Ibid. footnote 27.
45
Jane Horvath. State Legislatures Start Out Strong in 2018 with Bills to Curb Rx Drug Costs. NASHP Blog,
January 23, 2018. https://nashp.org/state-legislatures-start-out-strong-to-curb-rx-costs-in-2018/ Accessed
April 27, 2018.
46
Hannah Brennan, Amy Kapczynski, Christine H. Monahan, and Zain Rizvi. A Prescription for Excessive
Drug Pricing: Leveraging Government Patent Use for Health. Yale J.L. & Tech 2016; 275(18): 275- 354.
https://www.yjolt.org/sites/default/files/kapczynski_18yjolt275_gk_0_0.pdf. Accessed April 27, 2018.
47
Katherine Young and Rachel Garfield. Snapshots of Recent State Initiatives in Medicaid
Prescription Drug Cost Control. Henry J Kaiser Family Foundation. February 2018. http://files.kff.org/
attachment/Issue-Brief-Snapshots-of-Recent-State-Initiatives-in-Medicaid-Prescription-Drug-Cost-Control.
Accessed April 27, 2018.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
28

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
48
Between May 8 and June 7, 2017, Louisiana Department of Health was reviewing public comment
received on using 28 U.S.C. §1498 to buy generic versions of Hepatitis C drugs. While there were a
number of powerful statements from patients, family members, health care professionals, other Louisiana
residents, and national advocacy groups highlighting the burden of Hepatitis C and expressing their
support for this approach, the LDH also received opposing statements from the pharmaceutical industry
and other stakeholders without offering an alternative approach. LDH is continuing to consult with
additional stakeholders for next steps moving forward. http://ldh.la.gov/index.cfm/newsroom/detail/4227.
Accessed April 27, 2018.
49
Henry Waxman, Bill Corr, Kristi Martin, Sophia Duong. Getting to the Root of High Prescription Drug:
Prices Drivers and potential solutions. The Commonwealth Fund. July 2017. http://www.
commonwealthfund.org/~/media/files/publications/fund-report/2017/jul/waxman_high_drug_prices_drivers_
solutions_report.pdf. Accessed April 27, 2018.
50
Aaron S. Kesselheim, MD, JD, MPH; Michael S. Sinha, MD, JD, MPH; Jerry Avorn, MD. Determinants of
Market Exclusivity for Prescription Drugs in the United States. JAMA Intern Med. 2017;177(11):1658-
1664. doi:10.1001/jamainternmed.2017.4329. Accessed April 27, 2018.
51
Juliette Cubanski and Tricia Neuman. Searching for Savings in Medicare Drug Price Negotiations. Henry J
Kaiser Family Foundation. April 2018. http://files.kff.org/attachment/issue-brief-searching-for-savings-in-
medicare-drug-price-negotiations. Accessed April 2018.
52
National Conference of State Legislature (NCSL). Pharmaceutical Bulk Purchasing: Multi-state and
Inter-agency Plans. November 2017. http://www.ncsl.org/research/health/bulk-purchasing-of-prescription-
drugs.aspx#SSDC. Accessed April 27, 2018.
53
ibid. footnote 36
54
Lucas Allen. Demanding Affordable Medicines: Lessons from Maryland’s Law against Price Gouging.
Truthout, September 18, 2017. http://www.truth-out.org/news/item/41966-demanding-affordable-
medicines-lessons-from-maryland-s-law-against-price-gouging. Accessed April 27, 2018.
55
Association For Accessible Medicines v. Brian E. Frosh, in his official capacity as Attorney General for the
State of Maryland; Dennis R. Schrader, in his official capacity as Secretary of the Maryland Department of
Health. U.S. Memorandum Of Law In Support Of Plaintiff’s Motion For Preliminary Injunction. Court Of
Appeals For The Fourth Circuit. Appeal from the United States District Court for the District of Maryland,
at Baltimore. Marvin J. Garbis, Senior District Judge. (1:17-cv-01860-MJG). 2018. http://www.ca4.
uscourts.gov/Opinions/172166.P.pdf. Accessed April 27, 2018.
56
Association For Accessible Medicines v. Brian E. Frosh. Petition For Rehearing En Banc. On Appeal from
the United States District Court for the District of Maryland (Marvin J. Garbis, District Judge) http://www.
marylandattorneygeneral.gov/news%20documents/AAMvFrosh_Petition_for_Rehearing.pdf. Accessed April
30, 2018
57
The National Academy for State Health Policy. Model Act: An Act to Promote Prescription Drug Price
Transparency and Cost Control. 2017. https://nashp.org/wp-content/uploads/2017/03/Transparency-Model-
Legislation.pdf. Accessed April 27, 2018.
58
Jason A. Helgerson. Medicaid Drug Cap Webinar. New York Department of Health. August, 2017. https://
www.health.ny.gov/health_care/medicaid/regulations/global_cap/docs/2017-8-29_medicaid_drug_cap.pdf.
Accessed April 27, 2018.
59
American Medical Association. AMA Calls for Ban on DTC Ads of Prescription Drugs and Medical Devices.
Statement released on November 17 2015. https://www.ama-assn.org/content/ama-calls-ban-direct-
consumer-advertising-prescription-drugs-and-medical-devices. Accessed April 27, 2018.
60
S.2623 - Protecting Americans from Drug Marketing Act. 114th Congress (2015-2016). https://www.
congress.gov/bill/114th-congress/senate-bill/2623/text. Accessed April 27, 2018.
61
Community Catalyst. Fact Sheet: Academic Detailing: Evidence-Based Prescribing Information. Pew
Prescription Project. April 2009. https://www.communitycatalyst.org/doc-store/publications/Academic_
Detailing_Fact_Sheet.pdf. Accessed April 27, 2018.
62
Wendy Goldstein, Sarah K. Giesting and Amy Dow. Vermont Bans Gifts and Expands Disclosure
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
29

Addressing Out Of Control Prescription Drug Prices Federal and State StrategiesAddressing Out Of Control Prescription Drug Prices Federal and State Strategies
Requirements for Payments to Health Care Providers. Epstein Becker Green. October 2009. https://www.
ebglaw.com/content/uploads/2014/06/32060_HealthAlertVermont.pdf. Accessed April 27, 2018.
63
National Conference of State Legislatures. NCSL Briefs for State Legislature: Health Cost Containment
and Efficiencies. May 2011. http://www.ncsl.org/documents/health/introandbriefscc-16.pdf. Accessed
April 27, 2018.
64
Ibid. footnote 34
65
Consumer representatives to the NAIC. Promoting Access to Affordable Prescription Drugs: Policy Analysis
and Consumer Recommendations for State Policymakers, Consumer Advocates, and Health Care
Stakeholders. August 2016. http://consumersunion.org/wp-content/uploads/2016/08/Promoting-Access-to-
Affordable-Prescription-Drugs_Aug-2016.pdf. Accessed April 27, 2018.
66
Federal Trade Commission. Authorized Generic Drugs: Short-Term Effects and Long-Term Impact. August
2011. https://www.ftc.gov/sites/default/files/documents/reports/authorized-generic-drugs-short-term-
effects-and-long-term-impact-report-federal-trade-commission/authorized-generic-drugs-short-term-effects-
and-long-term-impact-report-federal-trade-commission.pdf. Accessed April 27, 2018.
67
Claire Laporte. Manufacturers of Biosimilar Drugs Sit Out the ‘Patent Dance’.” Health Affairs Blog, March
10, 2017. 10.1377/hblog20170310.059143. Accessed April 27, 2018.
68
Sarah Zhang. How Pharma Companies Use ‘Citizen Petitions’ to Keep Drug Prices High. The Atlantic,
March 8, 2017. https://www.theatlantic.com/health/archive/2017/03/pharma-citizen-petitions-drug-
prices/518544/. Accessed April 27, 2018.
69
Aaron S. Kesselheim and Jonathan J. Darrow. Hatch-Waxman Turns 30: Do We Need a Re-Designed
Approach for the Modern Era? Yale Journal of Health Policy, Law, and Ethics. 2015: Vol. 15: Issue 2,
Article 2. http://digitalcommons.law.yale.edu/yjhple/vol15/iss2/2. Accessed April 27, 2018.
70
Ramsey Baghdadi. Health Affairs Policy Brief: Medicaid Best Price. Health Affairs, August 10, 2017. doi:
10.1377/hpb2017.8. Accessed April 27, 2018.
71
The Pew Charitable Trusts. Issue Brief: GAIN: How a New Law Is Stimulating the Development of
Antibiotics. November 07, 2013. http://www.pewtrusts.org/en/research-and-analysis/issue-
briefs/2013/11/07/gain-how-a-new-law-is-stimulating-the-development-of-antibiotics. Accessed April 27,
2018.
72
Health Policy Alternatives, Inc. Prescription Drug Coverage for Medicare Beneficiaries: An Overview of the
Medicare Prescription Drug, Improvement, and Modernization Act of 2003. The Henry J. Kaiser Family
Foundation. January 2004. https://kaiserfamilyfoundation.files.wordpress.com/2013/01/prescription-drug-
coverage-for-medicare-beneficiares-an-overview-of-the-medicare-prescription-drug-improvement-act-2003.
pdf. Accessed April 27, 2018. Marsha Gold. Medicare Part D’s Importance Extends Far Beyond the Drug
Benefit It Provides. Health Affairs Blog, January 15, 2016. doi: 10.1377/hblog20160115.052694.
Accessed April 27, 2018.
73
U.S. Food & Drug Administration. Orange Book Preface: Food and Drug Administration Center for Drug
Evaluation and Research Approved Drug Products with Therapeutic Equivalence Evaluations. Preface to
the 38th Edition. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm079068.htm. Accessed
April 27, 2018.
74
Sarah Jane Tribbe and Sydney Lupkin Drugmakers Manipulate Orphan Drug Rules To Create Prized
Monopolies. Kaiser Health News, January 17, 2017. https://khn.org/news/drugmakers-manipulate-orphan-
drug-rules-to-create-prized-monopolies/. Accessed April 27, 2018.
75
Community and U.S. PIRG. Top Twenty Pay-For-Delay Drugs: How Drug Industry Payoffs Delay Generics,
Inflate Prices and Hurt Consumers. July 2013. https://www.communitycatalyst.org/doc-store/publications/
top-20-pay-for-delay-drugs.pdf. Accessed April 27, 2018.
76
Michael Carrier and Steve Shadowen. Pharmaceutical Product Hopping: A Proposed Framework for
Antitrust Analysis. Health Affairs Blog, June 1, 2017. doi: 10.1377/hblog20170601.060360. Accessed
April 27, 2018.
77
Michael A. Carrier. Sharing, Samples, and Generics: An Antitrust Framework. Cornell Law Review, Volume
103: Issue 1. November 2017. https://scholarship.law.cornell.edu/cgi/viewcontent.
cgi?article=4742&context=clr. Accessed April 27, 2018.
Addressing Out Of Control Prescription Drug Prices Federal and State Strategies
May 2018 | Addressing Out Of Control Prescription Drug Prices Federal and State Strategies | Community Catalyst
30

One Federal Street / Boston MA, 02110 / (617) 338-6035 / www.communitycatalyst.org / @HealthPolicyHub
